|
Number of Rare Codons
In this part we want to explore the influence of the number of rare codons inserted in the mRNA. We have inserted 2, 4, 6, 8 AGG codons respectively after the start codon in luciferase gene. T7 promoter or bla promoter[1] are used to control target protein mRNA amount. We use different combinations of number of AGG codons and strength of promoters to characterize regulation[1].
1) bla promoter-luciferase (weaker promoter)
A tandem of 2, 4, 6 or 8 AGG codons is inserted after the ATG codon of wild type luciferase
2) T7 promoter-luciferase (stronger promoter)
A tandem of 2, 4, 6 or 8 AGG codons is inserted after the ATG codon of wild type luciferase
Influence of inserted AGG codon number
The influence of different number of rare codons in regulating protein biosynthesis is shown below:
缺合体图
This picture reflects more clearly that the more rare codons are inserted, the lower the background expression and the narrower the range of device regulation. We are able to predict the outcome of influence of different number of rare codons in protein biosynthesis, offering valuable information for device usage.
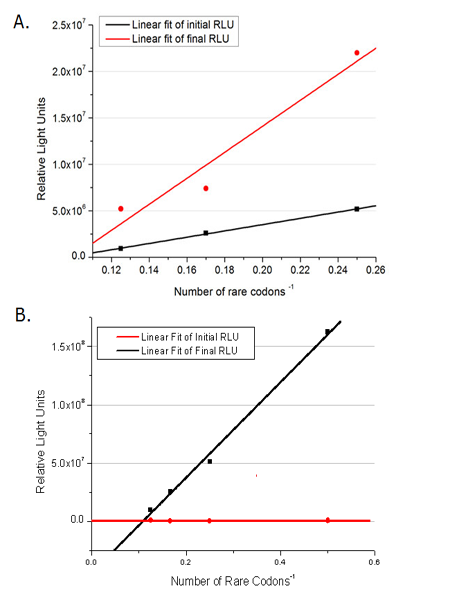 Fig.2 The comparison between background expression and induced expression of luciferase with different rare codon insertions. (A)PT7-luc reporters. (B) Pbla-luc reporters Influence of different strengths of target protein promoters
We examined the influence of different Reporter promoters on the working curve of our device, which is reflected by luciferase activity. The working range of our device is pre-defined by the strength of target protein promoter, T7 promoter and bla promoter in our project.
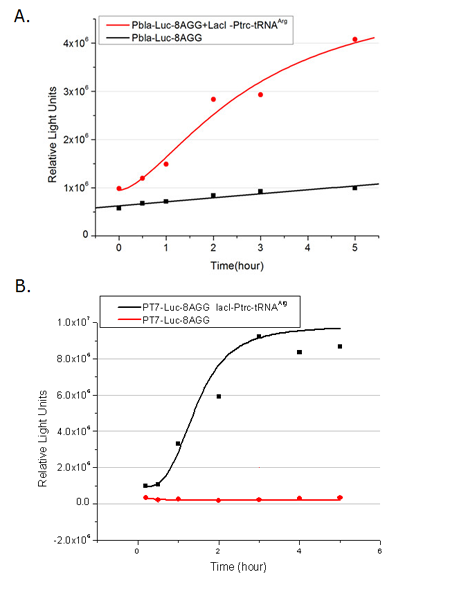 Fig.3 (A) Working curve of tRNA Modulator lacI-Ptrc-tRNAArg under Reporter Pbla-Luc-8AGG reflected by bioluminescence emitted from the luciferin reaction. (B) Working curve of tRNA Modulator under Reporter PT7-Luc-8AGG. Here we analyze the influences of strong/weak promoter in the working curve of tRNA Modulator. Moreover, strong promoter (T7) of target gene can improve the titration curve of tRNA Modulator, indicating that tRNA Modulator works better under strong target protein promoters. Note: Our device can be used as a regulating tool
We have tested luciferin reaction in cells. We examined the changes in luciferase enzyme activity over time after rare tRNA expression is induced. The amount of luciferase is reflected indirectly by the bioluminescence emitted from the luciferin reaction. Results are shown below:
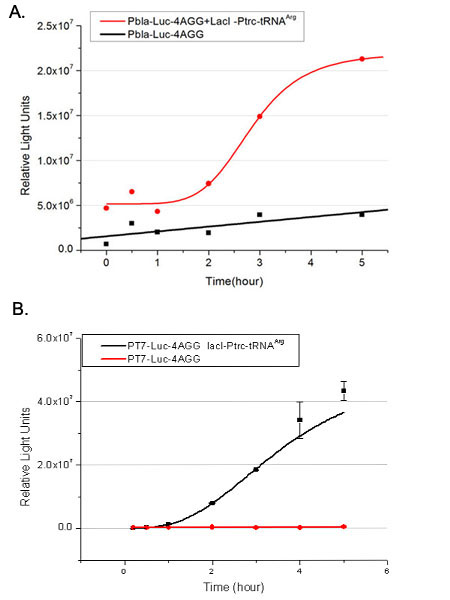 Fig.4 (A)Enzyme activity of luciferase shown by bioluminescence emitted from the luciferin reaction reflecting the working curve of tRNA Modulator lacI-Ptrc-tRNAArg ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567001 BBa_K567001]) under Reporter Pbla-Luc-4AGG([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567006 BBa_K567006]). (B)Enzyme activity of luciferase shown by bioluminescence emitted from the luciferin reaction reflecting the working curve of tRNA Modulator lacI-Ptrc-tRNAArg ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567001 BBa_K567001]) under Reporter PT7-Luc-4AGG ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567009 BBa_K567009]). The working curve of tRNA Modulator lacI-Ptrc-tRNAArg fits typical titration curve. Here we use the above two curves as examples to characterize the working curve of our device. Both curves fits typical titration curve, indicating that our device can function as a regulating tool.
The rest of the working curves are shown here:
Note:Click to see large figures.
Results showed that all the devices’ working curves fit titration curve, indicating that our device can act as a satisfying regulating tool.
From this experiment, we noticed that the typical working curve of our device can be better observed under IPTG induced lacI-Ptrc-tRNAArg ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567001 BBa_K567001]) compared with UV excitation induced sulA promoter-tRNAArg([http://partsregistry.org/wiki/index.php?title=Part:BBa_K567002 BBa_K567002]), though sulA promoter-tRNAArg responded quicker to signals. So in the above experiments, we test with lacI-Ptrc-tRNAArg.
Location of Rare Codons
We further study the influence of AGG codon in regulating protein biosynthesis when they are placed at different locations with different copy numbers. We designed three constructs to test the two influencing factors.
A tag with two-copy 4AGG insertions with an interval of 30 bp was before the luciferase gene
part fig
A tag with two-copy 4AGG insertions with an interval of 111 bp was added before the luciferase gene
part fig
A tag with three-copy 4AGG insertions with intervals of 30 bp and 111bp was added before the luciferase gene
part fig
Experiment results showed that the length of interval between the two-copy 4AGG did not affect much of the background production.
柱状图
Yet, protein production can be induced to a higher level when there is a longer interval (111bp).
曲线图+柱状图
When there are three copies of 4AGG in the tag, background production is lowered. The yield is also lowered.
Experiment results showed that luciferase tagged with two-copy 4AGG insertions with an interval of 111 bp can increase yield and lower background noise of target protein.
When we compare the two-copy 4AGG tagged luciferase with the single copy one, we find that two-copy 4AGG tagged luciferase has a higher yield.
When we compare the two-copy 4AGG tagged luciferase with 8AGG tagged luciferase, we find that two-copy 4AGG tagged luciferase has a lower background.
|