Team:Glasgow/Ranaspumin2
From 2011.igem.org
| Line 32: | Line 32: | ||
<p> | <p> | ||
| - | Coded for by the gene <i>rsn-2</i>, | + | Coded for by the gene <i>rsn-2</i>, Ranaspumin2 (Rsn2) is a monomeric surfactant protein, with an atomic mass of 11kDa. It has a molecular weight of 13415.2 and comprises of 117 amino acids. In aqueous solution it adopts a tightly folded globular shape, which is uncharacteristic of surfactant proteins. In this 3D arrangement Rsn2 consists of a single helix over a four-stranded sheet, as can be seen in the image below. </p> |
| - | + | <img src="http://farm7.static.flickr.com/6155/6167506290_3278200261_m.jpg" alt="Ribbon structure of Ranaspumin2"/> | |
| - | + | <p> <font size="1" font color="grey"> Image 1: Ribbons structure of Ranaspumin2 A: Frontal view B: 90 degree-rotation (Mackenzie, 2009) </font> </p> | |
| + | <p> | ||
| + | This structure is highly unusual for a protein with surfactant properties, and appears to convey no significant amphiphilicity. It is therefore believed that Rsn2 undergoes a significant conformational change when at the air-water interface, in which it will expose hydrophobic groups to the air and hydrophilic groups to the water beneath </p> | ||
| + | |||
| + | |||
| + | |||
</p> | </p> | ||
Revision as of 21:23, 20 September 2011

Ranaspumin2
Ranaspumin 2 is one of six proteins used by the tungara frog (Engystomops pustulosus, found in Central and South America) to form protein foam nests in which it deposits its eggs. Protein foams are relatively rare in biology, and in this case offer a biocompatible solution to incubation and protection from mechanical destruction, as well as anti-microbial properties.

A Tungara frog foam nest from Trinidad1 |
Structure
Coded for by the gene rsn-2, Ranaspumin2 (Rsn2) is a monomeric surfactant protein, with an atomic mass of 11kDa. It has a molecular weight of 13415.2 and comprises of 117 amino acids. In aqueous solution it adopts a tightly folded globular shape, which is uncharacteristic of surfactant proteins. In this 3D arrangement Rsn2 consists of a single helix over a four-stranded sheet, as can be seen in the image below.
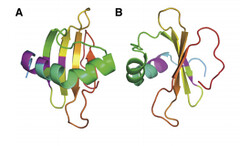
Image 1: Ribbons structure of Ranaspumin2 A: Frontal view B: 90 degree-rotation (Mackenzie, 2009)
This structure is highly unusual for a protein with surfactant properties, and appears to convey no significant amphiphilicity. It is therefore believed that Rsn2 undergoes a significant conformational change when at the air-water interface, in which it will expose hydrophobic groups to the air and hydrophilic groups to the water beneath
Click here for Ranaspumin2 sequence "
"
