Team:Cornell/Week 12
From 2011.igem.org
Week 1 | Week 2 - 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | Week 16 | Week 17 | Week 18 | Week 19 | Week 20 | Week 21 |
August 21st - August 27th
Sunday, August 21
Evening lab work done by: Youjin Cho, Charlie Chung
- Picking Colonies and Starting Up Cultures of VioB Ligation Reaction
- (AviTagged pZE12 backbone) control plate shows no growth of colonies
- (VioB + AviTagged pZE12 backbone) plate shows numerous colonies
- - Picked three colonies (VioB + AviTag + BB #1, 2, 3) and started 3mL cultures in LB with 3μL ampicillin
- - Incubated overnight in the 37°C shaker of Room 304
- PCR Reaction Setup of GFP (because sequencing results were negative)
- 38.5μL ddH2O
- 5μL 10x buffer
- 2.5μL dNTP
- 1μL forward GFP primer
- 1μL reverse GFP primer
- 1μL (pZE12+GFP) backbone template
- 1μL Vent DNA polymerase
- 50μL Total
- Annealing temperature of 54.2°C
- 1 minute extension cycle
Monday, August 22
Morning lab work done by: Youjin Cho
- Objective
- Miniprep the VioB + AviTag + pZE12 samples and submit for sequencing
- Miniprep and Sequencing
- Miniprepped the VioB + AviTag + pZE12 (1,2,3) samples using standard Qiagen Miniprep protocol
- Using 1μL of pZE12 reverse primer dilution and 17μL of each template, prepared and submitted the samples for sequencing.
- Order number: 10255430
Tuesday, August 23
Afternoon lab work done by: Claire Paduano, Maneesh Gupta, Youjin Cho, Charlie Chung
- Objective
- Set up new ligation of GFP and AviTagged backbone
- Send out gel purified GFP PCR reaction product to troubleshoot why our procedure isn't working
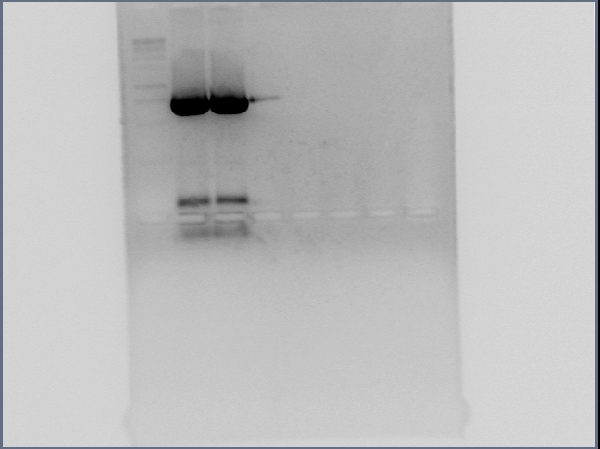
- Conducted gel electrophoresis on PCR reaction product of GFP
- - Visualization of migrated DNA fragments does not show a GFP band around 750bp
- Redid PCR on GFP
- - Note: This time, we made sure to add 1µL MgSO4
- 37.5μL ddH2O
- 5μL 10x buffer
- 1μL 25mM dNTP
- 1μL MgSO4
- 1μL forward GFP primer
- 1μL reverse GFP primer
- 1μL (pZE12+GFP) backbone template
- 1μL Vent DNA polymerase
- 48.5μL Total
- Annealing temperature of 54.2°C
- 1 minute extension cycle
- Gel electrophoresis
- - Visualization shows GFP band around 750bp
- - Visualization shows GFP band around 750bp
- Digestion Setups
- GFP insert
- 36μL H2O
- 6.5μL GFP (1μg)
- 5μL 10x NEBuffer 4
- 0.5μL 100x BSA
- 1.0μL KpnI-HF
- 1μL SphI-HF
- 50μL Total
- AviTagged pZE12 vector backbone
- 32.9μL H2O
- 9.6μL VioE in AviTagged pZE12 vector backbone (1μg)
- 5μL 10x NEBuffer 4
- 0.5μL 100x BSA
- 1.0μL KpnI-HF
- 1μL SphI-HF
- 50μL Total
- Incubate in 37°C water bath for 2 hours
Evening lab work done by: James Mathew, Maneesh Gupta, Claire Paduano
- Gel purified digested Avi-Tagged Backbone
- Ligation
- GFP + Avi-Tagged Backbone
- 5.8μL Avi-Tagged pZE12 vector backbone
- 7.97μL GFP
- 3.4μL H2O
- 2.0μL T4 DNA Ligase Buffer
- 1.0μL T4 DNA Ligase
- 20μL Total
- In above reaction, volume of plasmid vector added corresponds with 50ng backbone.
- Prepare control ligation reactions (inserts replaced with H2O) for each of the above constructs.
- Same volume of vector backbone, buffer, ligase
- Volumes of all inserts (i.e. gene) go toward volume of H2O
- After ligation setup, incubate at 16degC overnight.
Wednesday, August 24
Thursday, August 25
Lab work done by: Youjin Cho
- Transformation for GFP
- Desalted the ligation of GFP into AviTag + pZE12 backbone and the control on a membrane for 15 minutes
- Transformed the ligation GFP sample and control in DH5α electrocomptent cells via electroporation
- After shaking the cells in 37°C shaker for 50 minutes, plated onto agar treated with ampicilin and incubated overnight at 37°C
Friday, August 26
Morning lab work done by: Youjin Cho
- Checked the plates for GFP+Avitag+pZE12, but the ratio of ligation to control was bad.
Afternoon lab work done by: James Mathew, Maneesh Gupta, Claire Paduano
- Set up ligation for a second time since previous ligation was bad
- Ligation
- GFP + AviTagged Backbone
- 3.52μL AviTagged pZE12 vector backbone
- 7.97μL GFP
- 5.54μL H2O
- 2.0μL T4 DNA ligase buffer
- 1.0μL T4 DNA ligase
- 20μL Total
- In above reaction, volume of plasmid vector added corresponds with 50ng backbone
- Prepare control ligation reactions (inserts replaced with H2O) for each of the above constructs
- Same volume of vector backbone, buffer, ligase
- Volumes of all inserts (i.e. gene) go toward volume of H2O
- After ligation setup, incubate at 16°C overnight
Saturday, August 27
Weill Hall lab work done by: Claire Paduano, James Mathew
- Objective
- Coat microfluidic device with steptavidin.
- Run biotinylated fluorescent ATTO dye to test binding [ATTO dye info]
- Streptavidin coating and fluorescent labeling
- Protocol from Gleghorn et al: http://www.ncbi.nlm.nih.gov/pubmed/20024046
- 1) 45 min in 4% (by volume) MPTMS in ethanol
- 2) 20 min in 1mM GMBS
- 3) 45 min in 25ng/mL NeutrAvidin in PBS
- 4) 20 min (at flow rate 5ul/min) 1mM ATTO-590 dye
- Took photos of channel (bright field), negative control (streptavidin coated, no fluorescent probe), and streptavidin coated channel with fluorescent probe flushed through:
 "
"